Soil acidity is rapidly becoming a problem in our region. Each year more soil samples handled by the Noble Research Institute have or are prone to acidity problems. Acidic soils create production problems by limiting the availability of some essential plant nutrients and increasing that of the soil solution’s toxic elements, such as aluminum and manganese, the major cause of poor crop performance and failure in acidic soils. Below soil pH 5.5 (pH is the measurement of soil aciditythe lower the pH, the higher the soil acidity), aluminum may be concentrated enough to limit or stop root development. As a result, plants cannot absorb water and nutrients, are stunted, and exhibit nutrient deficiency symptoms (especially those for phosphorus). Toxic levels of manganese interfere with normal growth processes in the aerial plant parts, which stunts the plant, discolors it, and causes poor yields.
What causes soil acidity?
Producers commonly ask this question of staff soil fertility and crops specialists. There are four major reasons for soils to become acidic: rainfall and leaching, acidic parent material, organic matter decay, and harvest of high-yielding crops. Wet climates have a greater potential for acidic soils. In time, excessive rainfall leaches the soil profile’s basic elements (calcium, magnesium, sodium, and potassium) that prevent soil acidity. Soils that develop from weathered granite are likely to be more acidic than those developed from shale or limestone. Organic matter decay produces hydrogen ions (H+), which are responsible for acidity (an ion is a positively or negatively charged element). Like that from rainfall, acidic soil development from decaying organic matter is insignificant in the short term. Harvest of high-yielding crops plays the most significant role in increasing soil acidity. During growth, crops absorb basic elements such as calcium, magnesium, and potassium to satisfy their nutritional requirements. As crop yields increase, more of these limelike nutrients are removed from the field. Compared to the leaf and stem portions of the plant, grain contains minute amounts of these basic nutrients. Therefore, harvesting high-yielding forages such as bermudagrass and alfalfa affects soil acidity more than harvesting grain does.
Nitrogen fertilizer has been blamed for the increase in soil acidity problems throughout the region. Yes, when ammoniacal fertilizer materials are applied to the soil, acidity is produced, but the form of nitrogen removed by the crop is similar to that found in fertilizer. In reality, nitrogen fertilizer increases soil acidity by increasing crop yields, thereby increasing the amount of basic elements being removed.
How is soil acidity corrected?
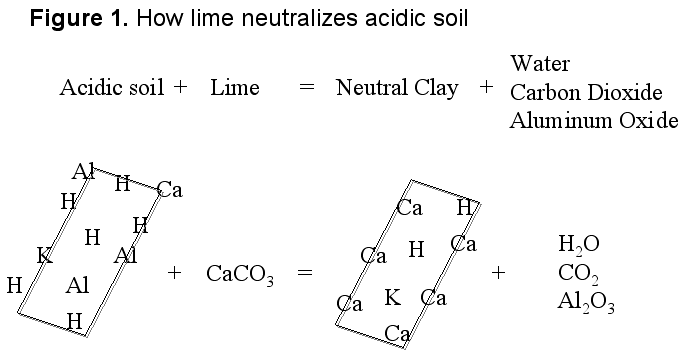
Soil acidity can be corrected easily by liming the soil, or adding basic materials to neutralize the acid present. The most commonly used liming material is agricultural limestone, the most economical and relatively easy to manage source. The limestone is not very water-soluble, making it easy to handle. Lime or calcium carbonate’s reaction with an acidic soil is described in figure 1, which shows acidity (H) on the surface of the soil particles. As lime dissolves in the soil, calcium (Ca) moves to the surface of soil particles, replacing the acidity. The acidity reacts with the carbonate (CO3) to form carbon dioxide (CO2) and water (H2O). The result is a soil that is less acidic (has a higher pH).
How much lime is needed?
Although harvested crops remove copious limelike elements each year, the soil pH does not change much from year to year, meaning the soil is buffered, or resistant to change.
The most important source of buffering in an acidic soil is the exchange of the limelike elementsmostly calciumattached to the surface of soil particles. As the crop removes these elements from the soil solution, attached elements move from the soil particles to replenish the solution. In time, reserve elements are depleted enough to cause acidity. When you apply lime, consider the size of the reservoir or buffering capacity. Typically, clay soils have a larger reservoir than sandy ones, which means that they require more lime to achieve a favorable pH. Pay attention to the buffer index or pH on the soil test because it is an indirect estimate of the soil reservoir’s size. Because the lab test involves adding basic material to soils with a pH less than 6.5 and then remeasuring pH, the buffer pH is larger when the reservoir is small (table 1). If the buffer pH is 6.8, then it will take 1.2 tons of effective calcium carbonate equivalent (ECCE) of lime to raise the pH to 6.8 and 0.7 ton to increase it to 6.4.
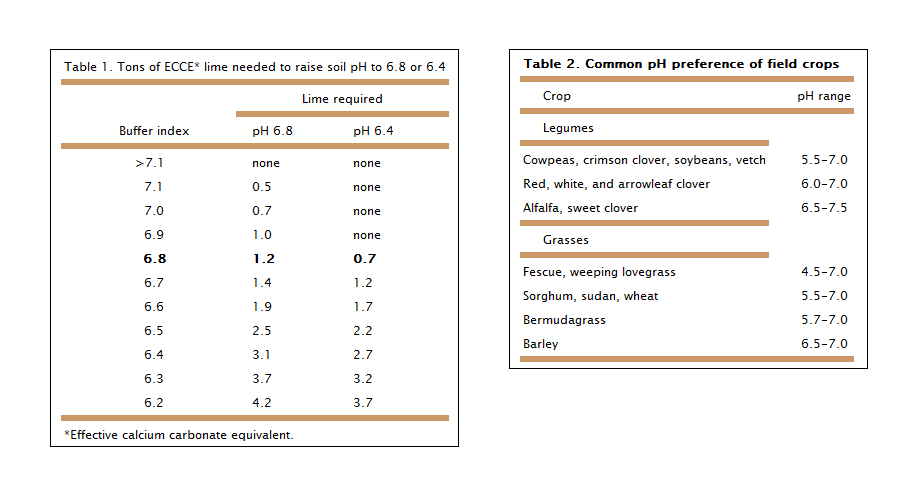
The correct pH depends on the crop being produced. Grasses tend to tolerate acidic soils better than legumes, so liming to pH 5.5 may control acidity without limiting production. Legumes, however, need more calcium and perform best between pH 6.5 and 7.5: pH 6.0 to 7.0 is best for nutrient availability. Table 2 indicates the pHs preferred by common field crops.
If you produce wheat continually (with no legume component), the minimum amount of lime to apply is 0.5 ton ECCE, or 25 percent of the soil test recommendation to raise the pH to 6.8.
Lime requirements are expressed in terms of ECCE, which is established on the basis of two components: the purity of the lime, determined chemically by the calcium carbonate content in the lime material, and the fineness of the lime material, determined by how much it is ground. The more calcium carbonate and the finer the material size, the higher the ECCE. Because the ECCE of lime is not always 100 percent, the amount of material required to provide that percentage must be calculated:
ECCE lime required x 100 = lime required ECCE %
How long will it take for lime to work?
It takes water to activate the lime reaction, so lime works slowly in dry soil. Even with adequate soil moisture, it may take a year or more for a measurable change in pH. Since neutralization involves a reaction between soil and lime particles, mixing lime with soil increases the efficiency of acidity neutralization. Test soil periodically when growing high-yielding perennial forages to identify lime deficiency early enough to change the pH with unincorporated broadcast applications.
Maintaining a favorable pH is extremely important in a soil fertility management plan. Routine soil testing reveals soil pH levels and provides liming recommendations. All too often, producers lose forage production by ignoring lime deficiency in soils with acidity problems.

Comment